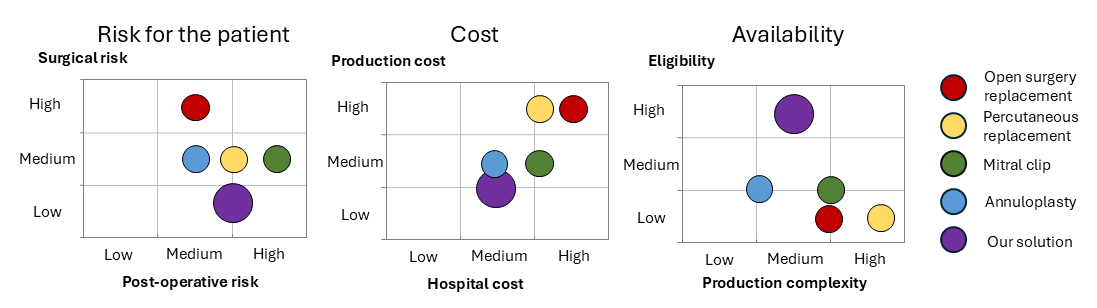
UNMET NEED
Mitral insufficiency is characterized by partial closure or non-closure of the mitral valve. This dysfunction leads to mitral regurgitation, with blood flowing back through the valve into the heart’s left atrium. Left untreated, mitral insufficiency results in pulmonary edema or cardiogenic shock, which can lead to the patient’s death. There are many treatment options and prostheses on the market, but 50% of the patients are not eligible for an open-heart surgery and percutaneous surgery is limited by the anatomical geometry of the valve to be replaced.
Even if the cardiac valve market is in growth (2 billion USD with a CAGR of 20%), 250 000 people have no options for mitral valve replacement in North America.